Abstract
Introduction
High-grade B-cell lymphomas are a subgroup of lymphomas characterized by high-grade morphological appearances and the presence of rearrangements in MYC in addition to BCL2 and/or BCL6 (HGBL-DH/TH) or in the absence of cytogenetic changes, HGBL-NOS, previously described as B-cell lymphoma unclassifiable with features intermediate between diffuse large B-cell lymphoma and classical Burkitt's lymphoma (BCLU). These entities have been shown to have poorer outcomes from conventional treatment compared with diffuse large B cell lymphomas (DLBCL) not otherwise specified. One feature contributing to the poor prognosis of DHL is increased risk of central nervous system (CNS) involvement. Intensive chemotherapy regimens which include CNS prophylaxis may improve PFS in HGBL compared with R-CHOP-like therapy.
Methods
We conducted an international, multi-center retrospective analysis of HGBL-DH/TH/NOS and BCLU. The association between baseline clinical and histopathological characteristics and clinical outcome was assessed by Cox proportional hazards model, and variables with P<0.10 by univariate analysis were included in multivariate Cox regression.
Results
57 patients were included - 20 patients with MYC/BCL2 rearrangements, 6 patients with MYC/BCL6 rearrangements, 13 with MYC/BCL2/BCL6 rearrangements, 16 with the WHO 2008 BCLU classification and 2 with HGBL-NOS classification. 16 (28%) patients had evidence of transformation from antecedent or synchronously diagnosed indolent lymphoma.
The median age at diagnosis was 63 years (range 39-86), with 30 (53%) male and median year of diagnosis was 2015 (range 2011 - 2017). The majority of patients were Stage III/IV [n=42, 74%] and ECOG ≤ 1 [n=44, 77%]. 40 patients (70%) had raised LDH at diagnosis; in 15 (26%) LDH was ≥3x ULN. Bone marrow involvement with high-grade lymphoma and extranodal involvement (excluding bone marrow) were present in 9 (16%) and 34 (60%) patients respectively. The median number of extranodal sites of involvement was 1 (range 0-10). Of 32 patients (56%) who underwent CNS staging at diagnosis, 2 (6%) had CNS involvement at baseline- one diagnosed with CSF cytology, and the other by combined modalities (CSF flow cytometry, cytology and MRI).
R-CHOP was the most common initial (49%) and ongoing (39%) induction chemotherapy regimen, followed by dose-adjusted EPOCH (32%), R-HyperCVAD and R-CODOX-M/IVAC (7% each) and other regimens (9%). A predominance of patients [n=33, 58%] received a form of CNS directed treatment- 8 (14%) as a standard component of the chosen induction regimen, and 25 (44%) as an addition to induction treatment (intrathecal [n=18, 32%], systemic therapy [n=2, 4%] or both [n=5, 9%]).
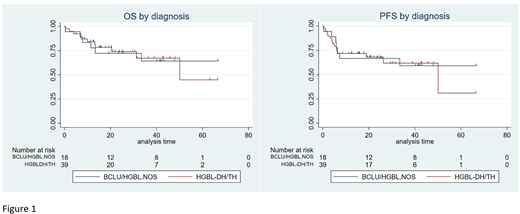
At a median follow-up of 25 months (range 5-67), the 2 year PFS and OS was 68% (95% CI: 50-81%) and 74% (95% CI: 55-85%) respectively for HGBL-DH/TH and 67% (95% CI: 40-83%) and 72% (95% CI: 46-87%) for BCLU/HGBL-NOS (Figure 1). Outcomes between the R-CHOP and intensive chemotherapy regimens were similar. At 2 years PFS was 70% (95% CI: 44-86%) vs 73% (95% CI: 52-86%), and OS was 79% (95% CI: 52-92%) vs 76% (95% CI: 56-89%). Two patients experienced CNS progression- one in a patient with BCLU and documented CNS disease at diagnosis, and another as a presentation of CNS relapse in a patient with HGBL-TH without evidence of CNS disease at diagnosis who did not receive specific CNS prophylaxis. Raised LDH was adversely prognostic for PFS in entire cohort and ECOG >2 was adversely prognostic for OS in the BCLU/HGBL-NOS group. Among patients who achieved a complete response at end of treatment, the 2 year PFS and OS was 96% (95% CI: 75-99%). In patients who had non-complete response (PD, SD, PR) the 2 year PFS and OS was 29% (95% CI: 13-48%) and 42% (95% CI: 22-62%) respectively. Among 23 patients (40%) with Deauville scores reported, those with Deauville score of 1-3 had a 2 year PFS and OS of 100%, with no events amongst 17 patients.
Conclusion
Within the limits of a retrospective analysis, outcomes of HGBL-DH/TH and HGBL-NOS/BCLU were similar. Rates of CNS involvement at diagnosis and on relapse were comparable with other retrospective series. Negative end of treatment PET-CT (DS 1-3) identifies a subset of patients with favorable prognosis.
Jeyasekharan:AstraZeneca: Other: Collaboration; Merck Sharp & Dohme: Honoraria; Janssen: Research Funding; PerkinElmer: Other: Collaboration. Bishton:Gilead: Research Funding; Abbvie: Research Funding; Roche: Research Funding; Takeda: Other: travel support to ASH.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal